Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
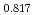 Pu
Pu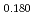 Am
Am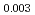 O
O uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 K
uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 KVauchy, R.; Sunaoshi, Takeo*; Hirooka, Shun; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Materials, 580, p.154416_1 - 154416_11, 2023/07
Times Cited Count:4 Percentile:97.05(Materials Science, Multidisciplinary)Hirooka, Shun; Kato, Masato; Watanabe, Masashi
Transactions of the American Nuclear Society, 118, p.1624 - 1626, 2018/06
This study suggested the time development of oxygen-to-metal ratio (O/M) redistribution model with oxygen-related properties in MOX. Irradiation simulation including the suggested O/M redistribution and pore migration with vaporization-condensation model which bares density redistribution was demonstrated. The simulation results showed that O/M redistribution proceeded at lower temperature than density redistribution, which indicated that oxygen diffusion got influential at lower temperature than vaporization-condensation of MOX. Another find was that O/M redistribution was very slow at the surface because temperature kept low. However, near the surface (inside from the surface) where the temperature exceeded 1000 K, O/M redistribution was rather recognizable with oxygen flown from inner region to the near-surface. The results will be evaluated by comparison with post-irradiation examination data.
 -CeO
-CeO
Hirooka, Shun; Murakami, Tatsutoshi; Nelson, A. T.*; McClellan, K. J.*
INL/EXT-14-33515, p.34 - 36, 2014/10
Collaborative study has been done on the properties of nuclear materials between the DOE and Japan and the oxygen potential of (U,Ce)O was measured in this year. Experimental measurements of the oxygen potential were conducted on Ce=20% and 30% composition rates simulating Pu content in advanced MOX fuel in JAEA by gas equilibrium method where oxygen partial pressure in the atmosphere was controlled with mixing dry/wet Ar/H
was measured in this year. Experimental measurements of the oxygen potential were conducted on Ce=20% and 30% composition rates simulating Pu content in advanced MOX fuel in JAEA by gas equilibrium method where oxygen partial pressure in the atmosphere was controlled with mixing dry/wet Ar/H gas. More than 100 data points were obtained in the O/M range of 1.945
gas. More than 100 data points were obtained in the O/M range of 1.945  2.000 at 1200
2.000 at 1200 C, 1400
C, 1400 C and 1600
C and 1600 C. The experimental results were analyzed by the defect analysis and analytical equations were obtained to calculate O/M as functions of temperature and oxygen potential. From the comparison with that of (U,Pu)O
C. The experimental results were analyzed by the defect analysis and analytical equations were obtained to calculate O/M as functions of temperature and oxygen potential. From the comparison with that of (U,Pu)O , applicability of the same defect chemistry and S-style curve are common. Also, it is revealed that (U,Ce)O
, applicability of the same defect chemistry and S-style curve are common. Also, it is revealed that (U,Ce)O requires evidently higher oxygen potential for the O/M.
requires evidently higher oxygen potential for the O/M.
; ;
JNC TN9400 2000-029, 87 Pages, 1999/11
The second Power-To-Melt (PTM) test, PTM-2, was performed in the experimental fast reactor "JOYO". AIl of the twenty-four fuel pins of the irradiation vehicle, B5D-2, for the PTM-2 test, were provided for post-irradiation-experiment (PIE) to evaluate the PTM values. ln this study, the PIE technique for PTM test was established and the PTM results were evaluated. The findings are as follows: (1) The maximum fuel-melting ratio on the transverse section was 10.7%, and was within the limit of fuel-melting in this PTM test enough. Unexpected fuel-melting amount to a ratio of 11.8% was found at  24 mm below the peak power elevation in a test fuel pin, lt is possible that this arose from secondary fuel-melting. (2) Combination of metallographical observation with X-ray microanalysis of plutonium distribution was very effective for the identification of once-molten fuel zone. (3) The PTM evaluation suggested that dependence of the PTM on the fuel pellet density was stronger than that of previous foreign PTM tests, while the dependence on the pellet-cladding gap and the oxygen-to-metal ratio was indistinctly. The dependence on the cladding temperature and the fill gas composition was not shown as well.
24 mm below the peak power elevation in a test fuel pin, lt is possible that this arose from secondary fuel-melting. (2) Combination of metallographical observation with X-ray microanalysis of plutonium distribution was very effective for the identification of once-molten fuel zone. (3) The PTM evaluation suggested that dependence of the PTM on the fuel pellet density was stronger than that of previous foreign PTM tests, while the dependence on the pellet-cladding gap and the oxygen-to-metal ratio was indistinctly. The dependence on the cladding temperature and the fill gas composition was not shown as well.



 U
U


 O
O

 solid solution
solid solutionUgajin, Mitsuhiro; ; Shiba, Koreyuki
Journal of Nuclear Materials, 116(2-3), p.172 - 177, 1983/00
Times Cited Count:15 Percentile:81.67(Materials Science, Multidisciplinary)no abstracts in English


 solid solutions
solid solutionsUgajin, Mitsuhiro
Journal of Nuclear Materials, 110, p.140 - 146, 1982/00
Times Cited Count:24 Percentile:88.74(Materials Science, Multidisciplinary)no abstracts in English
; ; ;
Journal of Nuclear Materials, 102, p.40 - 46, 1981/00
Times Cited Count:146 Percentile:99.69(Materials Science, Multidisciplinary)no abstracts in English

Murakami, Tatsutoshi; Kato, Masato; Nelson, A.*; McClellan, K.*
no journal, ,
no abstracts in English
 thermal conductivity
thermal conductivityHirooka, Shun; Kato, Masato; Nelson, A.*; McClellan, K.*; White, J.*
no journal, ,
Thermal diffusivity of (U,Ce)O was measured as a function of O/M, and the thermal conductivity was calculated. Thermal conductivity of (U,Ce)O
was measured as a function of O/M, and the thermal conductivity was calculated. Thermal conductivity of (U,Ce)O decreased more sharply with increasing temperature compared with that of (U,Pu)O
decreased more sharply with increasing temperature compared with that of (U,Pu)O . Also, thermal conductivity of (U,Ce)O
. Also, thermal conductivity of (U,Ce)O showed the highest peak at O/M of 2 and decreased as the O/M is apart from 2 on both hypo-stoichiometric and hyper-stoichimetric regions.
showed the highest peak at O/M of 2 and decreased as the O/M is apart from 2 on both hypo-stoichiometric and hyper-stoichimetric regions.
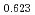 Pu
Pu Am
Am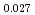 )O
)O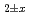 under high temperature
under high temperatureMatsumoto, Taku; Kato, Masato; Morimoto, Kyoichi; Sunaoshi, Takeo*
no journal, ,
The oxygen potentials of (U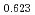 Pu
Pu Am
Am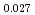 )O
)O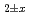 were measured as a function of oxygen to metal ratio (O/M) in the temperature range from 1673 to 1873 K by the gas equilibrium method using thermogravimetry. The measured oxygen potentials were analyzed by the point defect chemistry method. From this analysis, the oxygen potential of (U
were measured as a function of oxygen to metal ratio (O/M) in the temperature range from 1673 to 1873 K by the gas equilibrium method using thermogravimetry. The measured oxygen potentials were analyzed by the point defect chemistry method. From this analysis, the oxygen potential of (U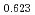 Pu
Pu Am
Am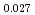 )O
)O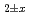 was represented as a function of O/M ratio and temperature.
was represented as a function of O/M ratio and temperature.
Kamei, Miho; Naganuma, Masayuki; Ikusawa, Yoshihisa; Maeda, Koji; Sasaki, Shinji; Ozawa, Takayuki; Hirooka, Shun
no journal, ,
no abstracts in English
Hirooka, Shun; Kato, Masato; Watanabe, Masashi
no journal, ,
no abstracts in English
Hirooka, Shun; Matsumoto, Taku; Kato, Masato; Sunaoshi, Takeo*
no journal, ,
Oxygen potentials of (U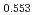 Pu
Pu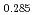 Am
Am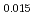 Np
Np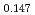 )O
)O pellet sintered in the atmosphere of Ar/H
pellet sintered in the atmosphere of Ar/H /H
/H O mixture gas were measured at 1600
O mixture gas were measured at 1600 C and 1650
C and 1650 C by the gas equilibrium method. The oxygen partial pressure of the atmosphere was accurately adjusted by changing H
C by the gas equilibrium method. The oxygen partial pressure of the atmosphere was accurately adjusted by changing H O content in the gas and monitored with oxygen sensors. Simultaneously, weight change originated by oxidation or reduction was measured by thermo-gravimetry method and O/M was calculated by the weight change. The measured data showed that the substitution of minor actinide with U increased the oxygen potential, indicating that O/M could be lowered by adding minor actinide if temperature and oxygen partial pressure of the surrounding gas was preserved. The present data was analyzed together with the previous Am-MOX data and led to the results that containing 1% of Am is equivalent to 4% of Pu on the effect of oxygen potential. Similarly, 1% of Np is equivalent to 0.2% of Pu. These effects were reflected to the defect formation energies and the relation among O/M, temperature, content ratio and oxygen partial pressure was derived. This relation can evaluate O/M of MA-MOX more accurately and, therefore, is the useful fuel technology for fuel design and evaluating irradiation behaviors.
O content in the gas and monitored with oxygen sensors. Simultaneously, weight change originated by oxidation or reduction was measured by thermo-gravimetry method and O/M was calculated by the weight change. The measured data showed that the substitution of minor actinide with U increased the oxygen potential, indicating that O/M could be lowered by adding minor actinide if temperature and oxygen partial pressure of the surrounding gas was preserved. The present data was analyzed together with the previous Am-MOX data and led to the results that containing 1% of Am is equivalent to 4% of Pu on the effect of oxygen potential. Similarly, 1% of Np is equivalent to 0.2% of Pu. These effects were reflected to the defect formation energies and the relation among O/M, temperature, content ratio and oxygen partial pressure was derived. This relation can evaluate O/M of MA-MOX more accurately and, therefore, is the useful fuel technology for fuel design and evaluating irradiation behaviors.
Nishina, Masahiro; Takato, Kiyoto; Nakamichi, Shinya; Segawa, Tomoomi; Kawaguchi, Koichi; Ishii, Katsunori; Makino, Takayoshi; Okumura, Kazuyuki
no journal, ,
no abstracts in English
阿部 雄太; 中桐 俊男
川上 智彦*
【課題】金属を主成分とする材料中のO、Bの組成や材料の硬度を非接触で精密に算出できる。 【解決手段】図5の関係より、上記の方法で算出された(B/O)/M値のみからビッカース硬度を推定することは一般的には困難である。しかしながら、試料が図5におけるZを挟んだどちらの領域にあるかを判定することができれば、(B/O)/M値からビッカース硬度を推定することができる。前記の通り、図5におけるZよりも左側の領域はOの組成比が大きい場合であり、Zよりも右側の領域はBの組成比が大きな場合に対応する。このため、前記のO/M値、B/M値に応じてこの試料がどちらの領域に属するかを認識することができる。結局、(B/O)/M値から前記の一次式を用いてビッカース硬度を算出することができる。